Which Direction of the Reaction Is Favored at 298 K
At a certain temperature bromine and nitric oxide react to form nitrosyl bromide. IF the temperature is raised then the reverse reaction is favored.
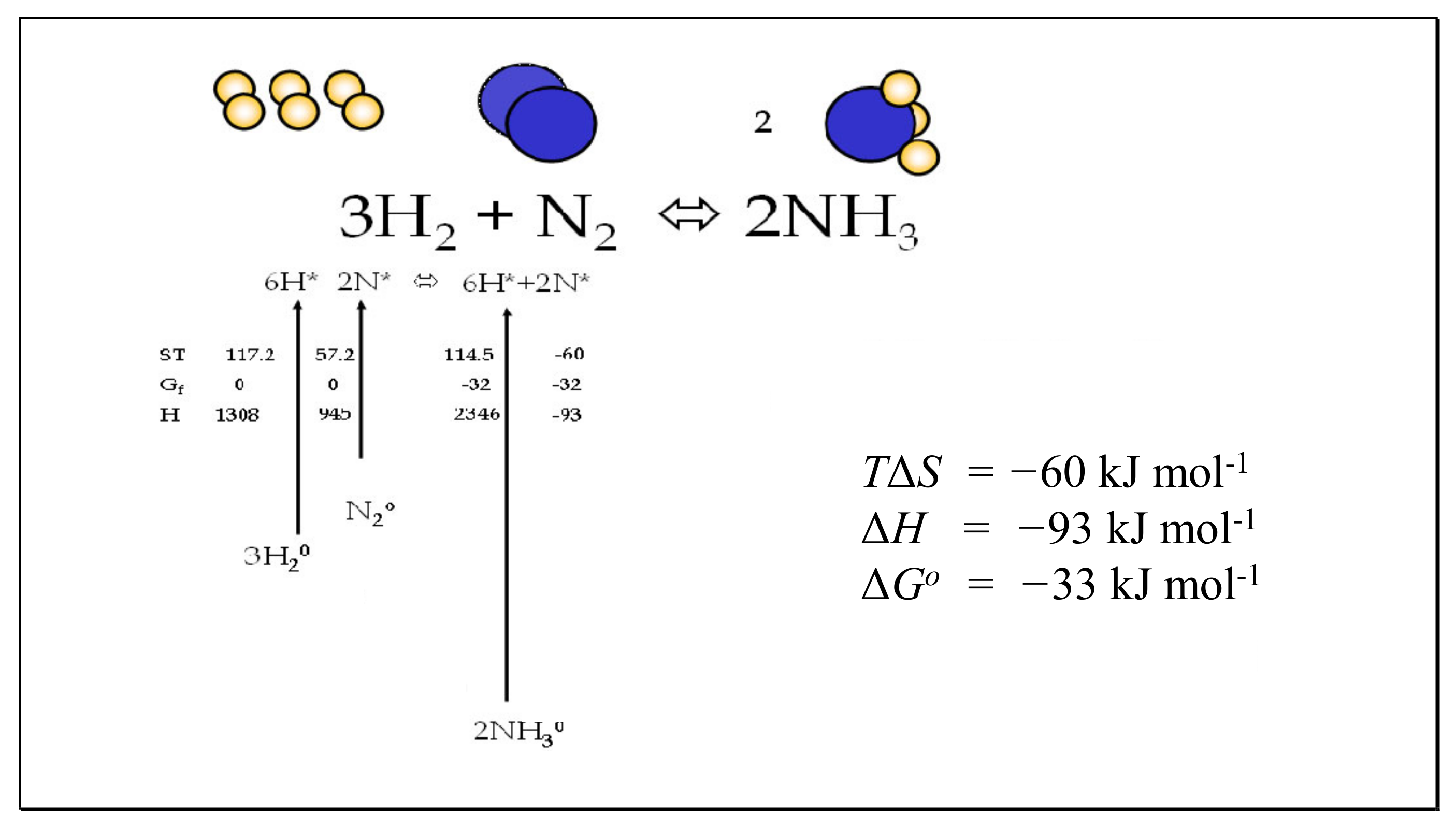
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html
Since we got a positive sign that means the reaction is non-spontaneous.
. The reverse reaction is favored C Neither direction is favored. In the appendix I have the following. Answer 1 Reactant favored.
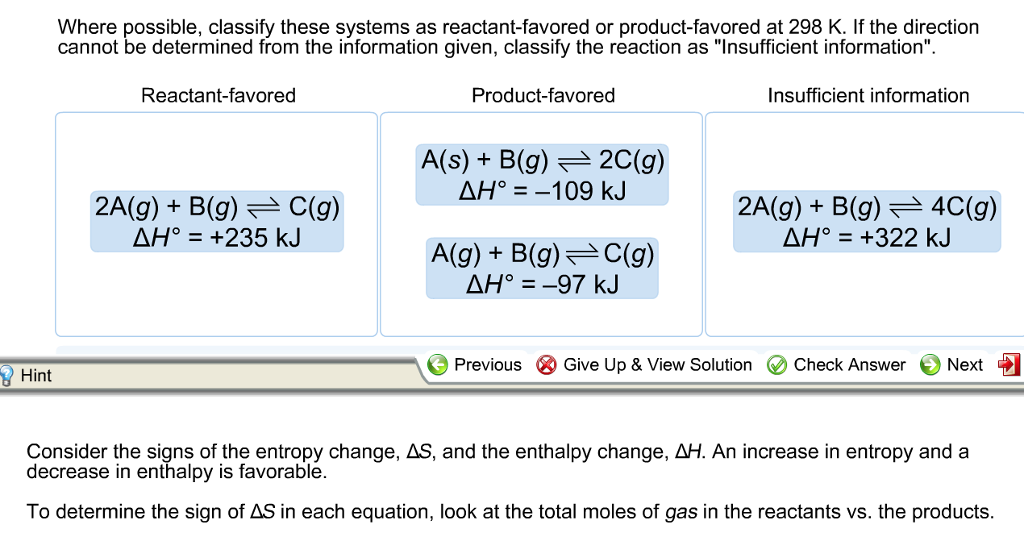
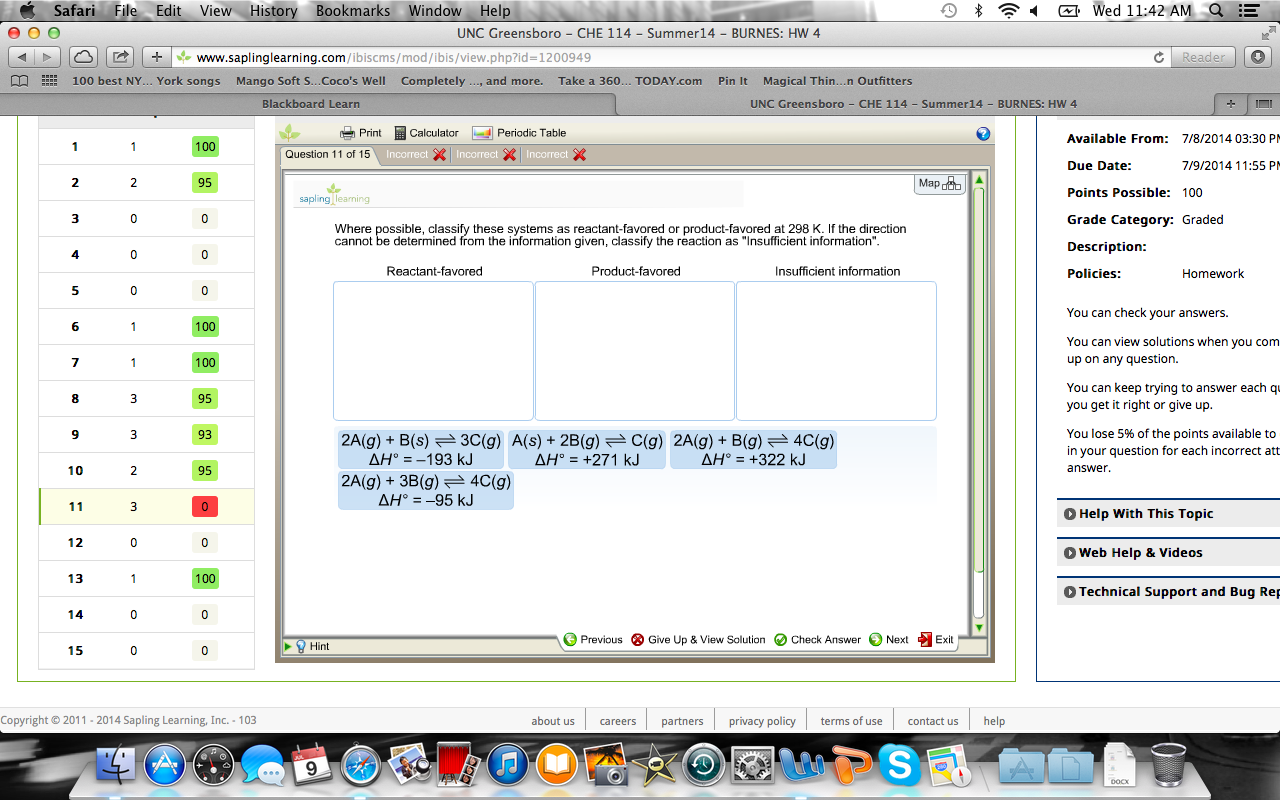
Answer 2 Reactant favored. 2 3 ---- 4 ΔH -95 KJ. The reaction will favor the product at this temperature.
Use the following data to answer questions 5-6. Never forget that it is. -372kJ An A __________ reaction liberates heat and the temperature of the reaction mixture increases.
1Gaseous species are at a pressure of 1 bar. Spontaneous changes occur only in the direction that leads to equilibrium. Consequently the reverse of this reaction would make it negative and spontaneous.
59 Which of the following reactions will have the largest equilibrium constant K at 298 K. G H- TS G 624 kJmol - 298 K 0145 kJmolK G 1919 kJ Now the sign of G is a criterion for spontaneity. Give the direction of the reaction if k 1.
ΔG 513 kJ at 298 K. 2The solution concentration is 1 molal. Calculate the Δ H and Δ S for this reaction at 298 K.
This gives 17 10 3 00412 0034 2x 017 x Multiplying both sides by 017 x gives. Consider the following reaction at 298 K. If its sign is - it means the reaction is spontaneous.
ΔS298 -73JKmol Which of the following statements about the thermodynamic favorability of the reaction at 298 K is correct. If the temperature is raised then the forward reaction favored. Give the direction of the reaction if k 1.
BR_2g 2 NOg. 2 2--- 3 ΔH 254 KJ. PCl3 g Cl2 g PCl5 g -8790kJ -01702kJK Calculate the Δ G for this reaction at 298 K using data from question 5.
IF the temperature is raised then the reverse reaction is favored. Which of the following statements concerning the spontaneity of chemical and physical changes is correct. A2A g 3B g --4C g delta H -95kJ b2A g 2B g --3C g delta H 254kJ c2A g B g -- 4C g delta H 322 kJ.
Next substitute the equilibrium concentrations into the Kc expression and solve for x. At a certain temperature bromine and nitric oxide react to form. Using data from Appendix 4 determine the equilibrium partial pressure of SO2 in the mixture.
The reverse reaction is favored C Neither direction is favored. It is easily observed that the reactant has 5 moles in total whereas the product has only 4 moles. This reaction is not spontaneous at any temperature.
Calculate Δ G 0 at 298 K for the reaction below. A CaCO3s CaOs CO2g ΔG 1311 kJ B 2 Hgg O2g 2 HgOs ΔG -1808 kJ C 3 O2g 2 O3g ΔG 326 kJ D Fe2O3s 3 COg 2 Fes 3 CO2g ΔG -280 kJ E It is not possible to determine without more information. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
When water is added to a mixture of Na2O2s and S s a redox reaction occurs as represented by the equation below. Kc 17 10 3 0034 2x2 017 x01 x Now take the square root of both sides of this equation. Where possible classify these systems as reactant-favored or product-favored at 298 K.
The forward reaction is favored. The formula is. Hence it is reactant favored reaction.
Entropy is positive and your enthalpy is also positive so this reaction will not be spontaneous at. The standard molar entropy change for the reaction at 298 K is 2875 Jmol-rxnK and the standard enthalpy change for the reaction at 298 K is -1368 kJmol-rxn 3NO 2 g H 2 Ol 2HNO 3 aq NOg a 511 kJmol b 85500 kJmol c 684 kJmol d 236 kJmol e 222 kJmol. If the temperature is raised then the forward reaction favored.
There fore the number of reactant is more than the products. 2 SO2g O2g 2 SO3g An equilibrium mixture contains O2g and SO3g at partial pressures of 050 atm and 20 atm respectively. The forward reaction is favored.
The ICE table looks like this. SO2g ΔGf -300kJmol. 3The temperature is 29815 K.
By using these guidelines we can quickly estimate whether a reaction will strongly favor the forward direction to make productsvery large strongly favor the backward direction to make reactantsvery small or somewhere in between. Note that the vertical offsets correspond to ΔH for the reaction. Reactants are favored and the reaction proceeds in the forward direction to reach equilibrium b Reactants are favored and the reaction is initially at equilibrium.
Which direction of the reaction equilibrium is favored at 298 K room temperature use the reaction I2 solid to I2 gas when H 624 kJmol and S 0145 kJmolK. 2 Na2O2s Ss 2 H2Ol 4 NaOHaq SO2aq ΔH298 -610 kJmol. The spontaneous direction of the reaction will always be in the direction in which the red shading overlaps the greater number of energy levels resulting in the maximum dispersal of thermal energy.
Solved Where Possible Classify These Systems As Chegg Com
Solved Where Possible Classify These Systems As Chegg Com

File Standard Electrode Potential Of Zinc Png Chemistry Physical Science Science

The University Arms Cambridge Brand Identity For Melford Abstract Artwork Brand Identity Artwork

Equilibrium Concpets Chemistry Education Chemistry Notes Chemistry Basics

Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

Pin By Johnetta On Current Mood In 2022 Tweet Quotes Current Mood Social Media

What Is The Final Potential Difference Across The Capacitor In 2022 Capacitor How To Apply Potential

Comments
Post a Comment